Struggling with equipment wear and failing coatings? These problems often stem from inferior chemical components. Isononanoic acid is the high-performance solution you need for ultimate durability and stability.
Isononanoic acid (INA) is a synthetic C9 carboxylic acid with a unique branched structure. This design provides exceptional thermal and hydrolytic stability, making it a key ingredient for high-performance polyol ester lubricants, durable low-VOC coatings, and advanced chemical applications where performance under stress is critical.
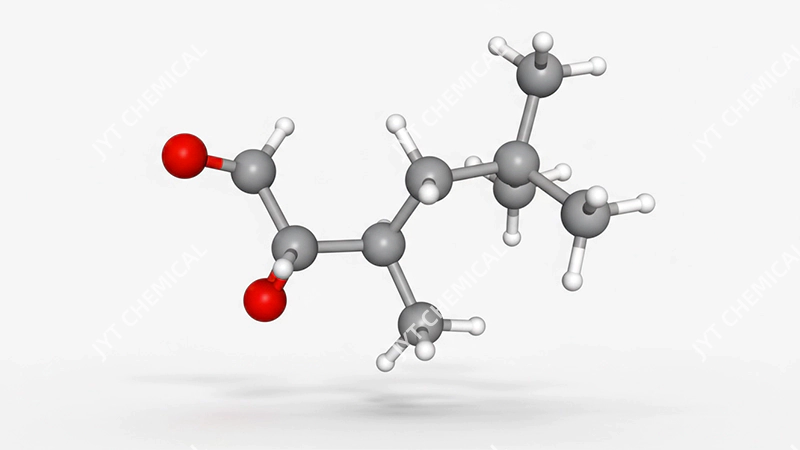
Molecular structure of isononanoic acid showing its branched shape.
As a formulator, I’m always searching for materials that provide a distinct performance advantage. Isononanoic acid is one of those special ingredients that consistently delivers. It’s not just another commodity chemical; it’s an engineered solution. In this pillar post, I’ll guide you through everything you need to know about this remarkable acid. We’ll start with the fundamentals of its chemistry, uncover why its structure is so advantageous, explore its critical roles in lubricants and coatings, and finally, discuss why it’s a safer, more sustainable choice for the future.
What Exactly is Isononanoic Acid?
Heard the term ‘C9 acid’ but unsure what it means? This chemical jargon can be confusing, preventing you from seeing its value. Let’s break it down simply.
Isononanoic acid is a synthetic, nine-carbon (C9) carboxylic acid, the typical isomer is 3,5,5-trimethylhexanoic acid. Its highly branched molecular structure, unlike linear fatty acids, is the source of its unique high-performance properties, making it a specialized chemical rather than a simple commodity fatty acid.

Diagram comparing the molecular structures of a branched isononanoic acid and a linear acid.
At its core, isononanoic acid is a member of the carboxylic acid family, which means its molecule contains a characteristic carboxyl functional group (-COOH). With the chemical formula C9H18O2, it is a colorless liquid that is soluble in most organic solvents. However, what truly sets it apart from many other acids used in industrial applications is its molecular architecture. While many common fatty acids have a straight, linear chain of carbon atoms, isononanoic acid is a synthetic compound with a highly branched structure. The most common isomer is 3,5,5-trimethylhexanoic acid, which has the CAS Number 3302-10-1. This isn’t an accident of nature; it’s a result of deliberate chemical synthesis designed to create specific performance characteristics. Think of it like the difference between a simple wooden plank and an engineered I-beam. Both are made of similar materials, but the I-beam’s structure gives it vastly superior strength and stability for demanding applications. The same principle applies here. This engineered, branched structure is the secret behind the acid’s remarkable properties, which we will explore in the next section.
The Significance of the Branched Structure
The "iso" or branched nature of isononanoic acid is the foundation of its value. This branching creates a bulky, three-dimensional molecule. Unlike long, straight-chain molecules that can easily stack together and crystallize, the awkward shape of isononanoic acid molecules prevents them from packing tightly. This physical disruption at the molecular level is directly responsible for its excellent fluidity at low temperatures. Furthermore, this bulkiness provides a protective effect, shielding the reactive parts of the molecule from chemical attack. This engineered structure is precisely why isononanoic acid is not just another C9 acid but a high-performance building block for creating materials that need to withstand extreme conditions, from the intense heat of a jet engine to the freezing cold of a refrigeration unit.
Why is Isononanoic Acid’s Branched Structure a Game-Changer?
Why choose one chemical over another? The molecular details seem abstract, but they directly impact your product’s real-world performance and lifespan. Let’s connect the dots.
The branched structure of isononanoic acid creates ‘steric hindrance,’ a molecular shield that provides three key benefits: exceptional thermal stability by preventing degradation, outstanding hydrolytic stability by blocking water, and excellent low-temperature fluidity by inhibiting crystallization. These properties are superior to linear acids.

Animation showing a bulky molecule acting as a shield to protect a chemical bond.
From my experience in formulation, understanding the "why" behind a material’s performance is crucial for innovation. With isononanoic acid, the "why" almost always comes back to its unique branched structure and the resulting phenomenon known as steric hindrance. This term simply means that the bulky, complex shape of the molecule gets in its own way, as well as in the way of other molecules. This "getting in the way" is not a flaw; it’s a powerful design feature that delivers a trifecta of stability advantages that are difficult to achieve with traditional linear acids. Let’s break down these three core benefits.
| Performance Advantage | Root Cause (Branched Structure) | Practical Benefit |
|---|---|---|
| Thermal & Oxidative Stability | Lack of beta-hydrogens, a common weak point for thermal degradation. | Resists breakdown at high temperatures, extending lubricant and coating life. |
| Low-Temperature Fluidity | Steric hindrance prevents molecules from packing neatly and crystallizing. | Products remain fluid and functional in cold environments. |
| Hydrolytic Stability | Steric hindrance shields the ester linkage from attack by water molecules. | Prevents degradation due to moisture, ensuring long-term reliability. |
Unmatched Thermal and Oxidative Stability
One of the primary failure mechanisms for many organic molecules, especially in high-heat applications, is a process called beta-hydrogen elimination. Linear acids possess these beta-hydrogens, which act as a chemical "eject button" when exposed to heat, initiating a chain reaction of degradation. The neo-acid structure of isononanoic acid, however, is engineered to lack these vulnerable beta-hydrogens. By removing this inherent weak point, the molecule becomes incredibly robust and resistant to breaking down under thermal stress. This is why polyol esters derived from isononanoic acid are the gold standard for applications like jet engine lubricants, where temperatures can reach extreme levels.
Superior Low-Temperature Fluidity
As mentioned earlier, the bulky, branched shape of isononanoic acid molecules prevents them from aligning neatly. When temperatures drop, linear molecules can easily stack together like logs, transitioning from a liquid to a solid. The awkward, three-dimensional shape of isononanoic acid disrupts this process. The molecules can’t find a way to pack efficiently, so they remain in a fluid state even at very low temperatures. This property is essential for refrigeration lubricants, which must continue to flow and lubricate compressors in sub-zero conditions.
Excellent Hydrolytic Stability
Hydrolysis is the chemical breakdown of a compound due to reaction with water. For esters, which form the backbone of many synthetic lubricants and resins, the ester linkage is a potential point of attack for water molecules. Here again, the steric hindrance of isononanoic acid acts as a protective shield. The bulky branched groups physically block water molecules from reaching and breaking the vulnerable ester bond. This results in lubricants and coatings that maintain their integrity and performance even in humid environments or where moisture contamination is a risk, significantly extending the operational life of the end product.
How Does Isononanoic Acid Elevate High-Performance Lubricants?
Your machinery needs lubricants that perform under pressure. Standard lubricants break down, causing costly failures. Discover the raw material behind the most resilient synthetic lubricants on the market.
Isononanoic acid is the essential building block for polyol ester (POE) synthetic lubricants. These POE lubricants, used in aviation, refrigeration, and industrial applications, offer superior thermal stability, low volatility, and excellent lubricity, directly thanks to the unique properties inherited from isononanoic acid.

Side-by-side images of a jet engine turbine and an industrial refrigeration compressor.
When we talk about isononanoic acid in the context of lubricants, we are primarily talking about its role in creating Polyol Esters, or POEs. These are top-tier Group V synthetic base oils, engineered for the most demanding environments. A POE is synthesized by reacting a polyol (an alcohol with multiple hydroxyl groups, like neopentyl glycol or trimethylolpropane) with a carboxylic acid. When isononanoic acid is used in this reaction, it imparts all of its signature benefits—thermal, oxidative, and hydrolytic stability—directly into the final lubricant. This makes POE lubricants derived from isononanoic acid the go-to choice for applications where mineral oils or other synthetic base oils would quickly fail. The result is less equipment downtime, longer service intervals, and enhanced operational reliability. For those looking to truly master the formulation of these advanced fluids, I’ve written a detailed guide. You can explore the chemical principles of optimizing engineered fluids with isononanoic acid in my dedicated post.
Aviation and Aerospace Lubricants
The environment inside a commercial jet turbine engine is one of the most extreme imaginable. Temperatures can swing from sub-zero at high altitudes to hundreds of degrees Celsius during operation. A lubricant here must not only reduce friction but also resist thermal decomposition, avoid vaporizing (low volatility), and act as a heat transfer fluid. POE lubricants based on isononanoic acid excel in this role. Their exceptional thermal and oxidative stability, derived directly from the acid’s branched structure, prevents the formation of sludge and carbon deposits that could cripple an engine.
Refrigeration and AC Compressor Oils
In refrigeration and air conditioning systems, the lubricant circulates with the refrigerant. It must remain fluid at very low temperatures to ensure it returns to the compressor, and it must be chemically stable, not reacting with the refrigerant or any trace moisture in the system. The excellent low-temperature fluidity and hydrolytic stability of INA-based POEs make them ideal for this purpose. They ensure consistent lubrication and system efficiency, which is critical for both residential AC units and large-scale industrial freezers.
What Role Does Isononanoic Acid Play in Modern, Eco-Friendly Coatings?
Stricter environmental regulations are making old coating formulas obsolete. Formulators are struggling to reduce VOCs without sacrificing performance. Isononanoic acid provides a powerful, compliant solution.
In coatings, isononanoic acid acts as a ‘chain stopper’ in alkyd resin synthesis, which helps lower viscosity and reduce the need for volatile organic compound (VOC) solvents. Its metal salts also serve as safer, more effective paint driers, improving durability and yellowing resistance.

A freshly painted wall with a ‘Low VOC’ label prominently displayed.
The coatings industry has been under immense pressure to become more environmentally friendly, with a primary focus on reducing Volatile Organic Compounds (VOCs). These solvents, which evaporate as paint dries, contribute to air pollution. From my perspective as a formulator, the challenge has been to lower VOCs without compromising the performance characteristics that customers expect, such as durability, gloss, and ease of application. Isononanoic acid has emerged as a key tool in achieving this balance, offering elegant solutions in both resin synthesis and paint drying. The innovations in this area are exciting, and for those who want to dive deeper, I recommend checking out my article on exploring innovative applications of isononanoic acid in eco-friendly, high-performance coatings.
Reducing VOCs in Alkyd Resins
Alkyd resins are a staple of the coatings industry, used in a wide range of enamels and primers. They are polymers, meaning they are made of long, repeating molecular chains. The length of these chains directly influences the resin’s viscosity; longer chains mean higher viscosity. To make a high-viscosity resin usable as paint, formulators have traditionally added large amounts of solvent (VOCs) to thin it down. This is where isononanoic acid comes in. When added during the resin synthesis, its bulky, monofunctional nature allows it to act as a "chain stopper." It attaches to the end of a growing polymer chain, preventing it from getting longer. By controlling the polymer chain length in this way, we can create an alkyd resin with inherently lower viscosity. This low-viscosity resin requires significantly less solvent to achieve the desired application consistency, leading directly to a low-VOC or even zero-VOC coating formulation.
Creating Safer and More Effective Paint Driers
For a coating to form a hard, durable film, it needs to cure or "dry." This process is accelerated by catalysts known as driers, which are typically metal salts of carboxylic acids. For decades, these driers were often made using 2-Ethylhexanoic acid (2-EHA). However, due to regulatory and toxicological concerns surrounding 2-EHA, the industry has been actively seeking safer alternatives. Metal salts derived from isononanoic acid have proven to be an excellent replacement. They are not only safer from a regulatory standpoint but also offer performance benefits. For instance, INA-based driers can lead to better long-term performance and improved resistance to yellowing in white and light-colored paints compared to driers based on traditional fatty acids.
Is Isononanoic Acid a Safer, More Sustainable Alternative?
Regulatory pressures are mounting against certain chemicals. Choosing the wrong raw material today can lead to costly reformulations tomorrow. Let’s examine the future-proof choice.
Yes, isononanoic acid is widely regarded as a safer and more sustainable alternative to chemicals like 2-Ethylhexanoic acid (2-EHA). Its favorable toxicological profile and classification mean it faces fewer regulatory restrictions, making it a more stable and forward-thinking choice for modern formulations.

A green checkmark symbol next to a chemical beaker labeled INA, signifying safety and approval.
In today’s chemical industry, performance is only part of the equation. Safety, regulatory compliance, and sustainability are equally important drivers of material selection. A product that performs well but carries a significant toxicological risk or is on the verge of being regulated out of existence is a poor long-term investment. From my professional standpoint, one of the most compelling reasons to adopt isononanoic acid is its superior safety profile compared to its main C8 competitor, 2-Ethylhexanoic acid (2-EHA). Making a proactive switch to a raw material with a better safety and regulatory outlook is one of the smartest strategic decisions a formulator or purchasing manager can make. It’s about de-risking your product line and building on a foundation of stability.
Navigating the Regulatory Landscape
The key difference lies in their regulatory classification, particularly in jurisdictions like the European Union. 2-EHA is classified under CLP regulations as a reproductive toxicant (Repr. 1B, H360D – "May damage the unborn child"). This classification triggers stringent handling requirements, labeling laws, and in many cases, outright bans for consumer applications. It places a significant burden on manufacturers and formulators who use it. In stark contrast, isononanoic acid (specifically 3,5,5-trimethylhexanoic acid) does not carry this classification. It has a much more favorable toxicological profile, which means it is not subject to the same severe restrictions. This makes INA a "future-proof" choice, insulating formulators from the risk of having to undertake costly and time-consuming reformulations should regulations on 2-EHA become even tighter. The differences between these two acids are critical for any formulator to understand, which is why I’ve created a head-to-head comparison in my post, Isononanoic Acid vs. 2-Ethylhexanoic Acid (2-EHA): Choosing the Right Carboxylic Acid for Your Formulation. Once you’ve decided on the right chemical, the next step is securing a reliable source. For guidance, see my checklist for sourcing an isononanoic acid supplier.
Conclusion
Isononanoic acid is more than just a chemical; it’s an enabling technology. Its unique branched structure delivers unmatched stability for lubricants and coatings. For formulators seeking performance, durability, and regulatory peace of mind, INA is an indispensable tool for creating the products of tomorrow.
References
-
- Isononanoic acid: jytchem.com.
-
- Industrial Isononanoic Acid Market: pmarketresearch.com.
-
- Industrial Grade Isononanoic Acid Market: sites.google.com.
-
- Carboxylic Acids: IUPAC Gold Book.
-
- Alkyd Resin: Wikipedia.