Choosing between INA and 2-EHA seems simple, but regulatory changes and performance demands complicate the decision. Making the wrong choice can lead to compliance issues and product failures.
Isononanoic acid (INA) is the superior choice for most new formulations due to its better thermal stability and favorable safety profile. 2-Ethylhexanoic acid (2-EHA), now classified as a reproductive toxicant, is being phased out in many applications in favor of safer alternatives like INA.
[Molecular models of Isononanoic Acid and 2-Ethylhexanoic Acid side-by-side]
For decades, 2-Ethylhexanoic acid (2-EHA) was a reliable workhorse for formulators. However, the landscape has changed dramatically. The choice between these two acids is no longer just a technical preference; it’s a critical business decision that impacts performance, safety, and long-term regulatory compliance. As a formulator, I’ve seen this shift firsthand, and understanding the deep-seated chemical differences is essential for future-proofing your products. Let’s break down this critical decision to see why isononanoic acid has emerged as the clear successor for modern, high-performance formulations.
At the Molecular Level: How Do INA and 2-EHA Structurally Differ?
Confused by the C8 vs. C9 distinction? Choosing the wrong carbon chain and structure can unknowingly compromise your lubricant’s thermal stability and low-temperature performance from the start.
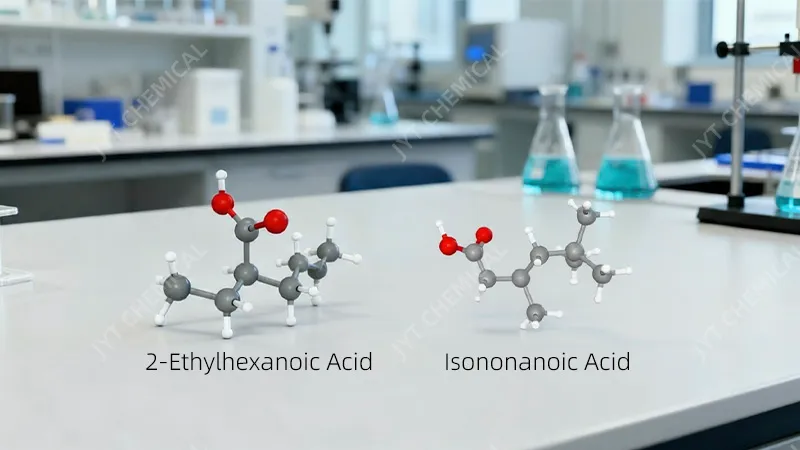
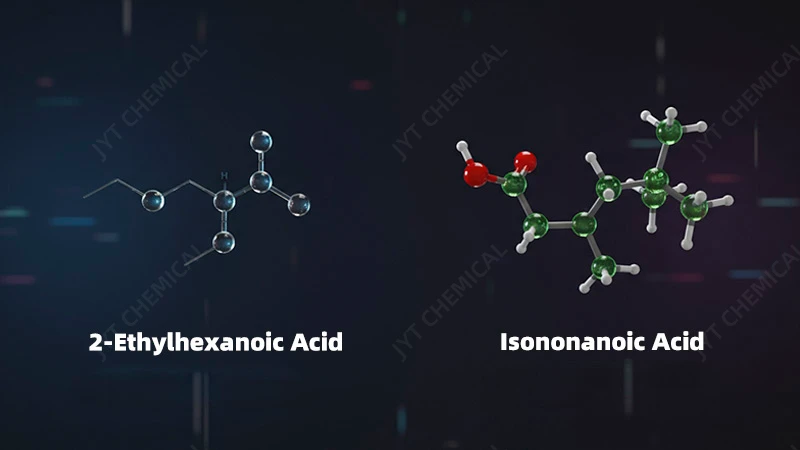
Isononanoic acid (INA) is a C9 branched acid (3,5,5-trimethylhexanoic acid), while 2-Ethylhexanoic acid (2-EHA) is a C8 branched acid. This extra carbon and different branching give INA a higher boiling point and, crucially, a structure that lacks beta-hydrogens, making it far more thermally stable.
[Diagram comparing the branched structures of INA and 2-EHA]
On the surface, INA and 2-EHA appear similar. Both are branched-chain carboxylic acids used to create esters, paint driers, and other chemical intermediates. However, their subtle structural differences lead to significant variations in performance.
The Core Structural Differences
- 2-Ethylhexanoic Acid (2-EHA): This is a C8 carboxylic acid with the chemical formula C8H16O2. Its key feature is a branch at the second carbon position (the “alpha-carbon”). This structure has served the industry well for years, providing good solubility for metal salts and decent performance in many esters.
- Isononanoic Acid (INA): This is a C9 carboxylic acid with the formula C9H18O2. It’s typically a mix of isomers, with the primary component being 3,5,5-trimethylhexanoic acid. Its branching is more complex and further down the carbon chain compared to 2-EHA.
The most critical difference, however, lies in the presence of beta-hydrogens. In 2-EHA, there are hydrogen atoms attached to the second carbon (the beta-carbon relative to the carbonyl group). In the primary isomer of INA, there are no hydrogens on the beta-carbon. As we will see, this single structural detail is the primary reason for INA’s vastly superior thermal stability. This seemingly small molecular detail has massive real-world consequences for the longevity and reliability of the final product.
| Feature | 2-Ethylhexanoic Acid (2-EHA) | Isononanoic Acid (INA) | Implication for Formulators |
|---|---|---|---|
| Carbon Chain | C8 | C9 | INA has a higher boiling point and molecular weight. |
| Structure | Branch at C2 position | Complex branching, e.g., at C3, C5 | INA’s bulkier structure improves low-temp fluidity in esters. |
| Beta-Hydrogens | Yes | No (in primary isomer) | INA has significantly higher thermal stability. |
The Regulatory Showdown: Why is 2-EHA’s Safety Profile a Major Concern?
Are you still using 2-EHA in your formulations? You could be exposing your company and customers to significant regulatory risk and future liability without even realizing it.
The European Chemicals Agency (ECHA) has reclassified 2-Ethylhexanoic acid and its salts as a "Reproductive Toxicant, Category 1B." This requires hazard labeling and is driving a global shift towards safer alternatives like isononanoic acid, which does not have this classification.

[A GHS health hazard pictogram on a chemical drum]
Perhaps the most compelling reason to switch from 2-EHA to INA has nothing to do with performance and everything to do with safety and regulation. The chemical industry is under constant scrutiny, and regulatory bodies are continually updating their classifications based on new toxicological data. According to a factsheet from ExxonMobil Chemical, the reclassification of 2-EHA as a Reprotoxic Category 1B substance by ECHA has sent shockwaves through the industry1. This classification means it is presumed to be a human reproductive toxicant.
Implications for Formulators
This isn’t just a bureaucratic label; it has severe, practical consequences for any company using 2-EHA:
- Mandatory Hazard Labeling: Products containing 2-EHA above a certain concentration must now carry the GHS health hazard pictogram and warning statements. This can be a major issue for consumer-facing products and can complicate logistics and handling.
- Stricter Worker Safety: Companies must implement more stringent handling procedures and provide additional personal protective equipment (PPE) for workers exposed to 2-EHA.
- Potential Bans and Restrictions: This classification puts 2-EHA on the radar for future restrictions or outright bans in certain applications, particularly those with high consumer contact. Relying on it is a significant business risk.
- Customer Perception: In today’s market, sustainability and safety are powerful purchasing drivers. A product labeled with a reproductive toxicity warning is at a major disadvantage.
In stark contrast, isononanoic acid has a much cleaner toxicological profile and is not subject to this classification. For any formulator developing a new product or reformulating an existing one, choosing INA is the clear path to ensuring long-term compliance, reducing liability, and aligning with market demands for safer chemistry.
Performance Under Pressure: Which Acid Creates More Thermally Stable Lubricants?
Your high-temperature lubricant is breaking down, forming sludge and deposits. This failure is likely rooted in the acid you chose, costing you in performance and equipment life.
Isononanoic acid creates significantly more thermally stable polyol esters than 2-EHA. This is because INA’s structure lacks beta-hydrogens, preventing a major thermal degradation pathway (beta-elimination) that 2-EHA is highly susceptible to.

[A split image showing a clean gear vs. a sludgy, coked gear]
While the regulatory aspect is critical, INA also delivers a clear performance upgrade, especially in high-temperature applications. The key lies in the beta-hydrogen we discussed earlier. The presence of these hydrogens in 2-EHA opens the door for a destructive reaction called beta-elimination. Under thermal stress, this "weak spot" allows the ester molecule to break apart, leading to the formation of volatile byproducts and the degradation of the lubricant. This process results in increased fluid acidity, viscosity changes, and the formation of harmful sludge and varnish that can cripple high-performance machinery.
Isononanoic acid, by its very design, shuts this door. Because its primary isomer has no beta-hydrogens, the beta-elimination pathway is blocked. This makes esters derived from INA inherently more resistant to breaking down under heat. From my own formulation experience, the difference is night and day. When running high-temperature oxidation tests, INA-based esters consistently show less viscosity increase, lower acid number buildup, and far fewer deposits compared to their 2-EHA counterparts. This superior thermal stability is not just a marginal improvement; it’s a fundamental shift in performance that translates directly into longer fluid life, extended drain intervals, and better protection for expensive equipment like jet turbines, industrial ovens, and high-output compressors. For any application where heat is a factor, INA is not just a safer choice—it’s the higher-performing one.
The Paint Drier Dilemma: Is INA a Viable Replacement for 2-EHA?
Your go-to paint drier based on 2-EHA is now a regulatory liability. You need a replacement that works just as well without forcing a complete reformulation of your paint system.
Yes, isononanoic acid is the industry’s leading replacement for 2-EHA in creating metal carboxylate paint driers. Isononanoates (e.g., cobalt isononanoate) offer comparable drying efficiency and solubility with a vastly superior safety profile, making the switch straightforward.

[A can of paint with a "2-EHA Free" label on it]
In the coatings world, the reclassification of 2-EHA has created a significant challenge. For decades, metal octoates (driers made with 2-EHA) have been the standard for catalyzing the drying process in alkyd paints. The acid’s role is to act as a carrier, making the metal catalyst (like cobalt or manganese) soluble in the oil-based paint. With 2-EHA now carrying a reproductive toxicity warning, formulators have been urgently seeking a "drop-in" replacement that doesn’t require a complete overhaul of their carefully balanced paint formulas.
Isononanoic acid has proven to be the perfect solution. Metal isononanoates perform this carrier function exceptionally well. The branched, nine-carbon structure of INA provides excellent solubility in the non-polar paint binder, ensuring the metal catalyst is dispersed effectively and can do its job. The performance is so similar to 2-EHA that in many cases, the substitution can be made with minimal adjustments to the rest of the formulation. This allows coatings manufacturers to quickly pivot away from the regulatory headache of 2-EHA without sacrificing the drying times and film properties their customers rely on. Furthermore, isononanoates often have a lower odor than octoates, which is an added benefit for both professional painters and DIY consumers. The move to INA-based driers is a clear win-win: it solves a critical regulatory problem while maintaining performance and even improving the product’s sensory characteristics.
Cost vs. Total Value: How Do INA and 2-EHA Compare Economically?
Is the slightly higher upfront cost of isononanoic acid holding you back? Focusing only on the per-kilogram price ignores the total cost of ownership, including compliance, performance, and risk.
While 2-EHA may have a lower initial purchase price, isononanoic acid provides a better total economic value. This is due to the reduced regulatory burden, superior performance leading to longer fluid life, and avoidance of potential liabilities associated with 2-EHA.
| Feature/Consideration | 2-Ethylhexanoic Acid (2-EHA) | Isononanoic Acid (INA) | Economic Implication for Formulators |
|---|---|---|---|
| Initial Purchase Price | Often lower per kg | Often slightly higher per kg | Short-term: 2-EHA seems cheaper. Long-term: INA offers better TCO. |
| Regulatory Burden | High (Reprotoxic Cat 1B) | Low (No reprotoxic classification) | 2-EHA: Significant costs for labeling, handling, compliance, and potential future restrictions. INA: Reduced regulatory risk and associated costs. |
| Performance (Thermal Stability) | Lower (due to beta-hydrogens) | Higher (no beta-hydrogens in primary isomer) | 2-EHA: Shorter product lifespan, more frequent maintenance, higher replacement costs in high-temp applications. INA: Longer product life, extended maintenance intervals, better equipment protection, reduced operational costs. |
| Risk & Liability | High (potential for lawsuits, recalls, bans) | Low (safer profile) | 2-EHA: Significant business risk, potential for catastrophic financial loss. INA: Acts as insurance against future regulatory changes and liability, ensuring business continuity. |
| Brand & Market Value | Negative (due to hazard labeling) | Positive (“Safer,” “2-EHA Free” differentiator) | 2-EHA: Damages brand reputation, limits market access, potential for customer rejection. INA: Enhances brand image, opens new markets, commands premium pricing, builds customer loyalty. |
| Total Cost of Ownership (TCO) | Higher (hidden costs outweigh initial savings) | Lower (benefits far outweigh initial premium) | 2-EHA: Apparent savings are eroded by compliance, performance, and risk costs. INA: Initial investment yields significant long-term savings and competitive advantages. |
It’s true that, on a per-kilogram basis, 2-EHA can sometimes be less expensive than INA. It’s a C8 acid, which is generally a less complex molecule to produce than a C9 acid. However, a smart formulator and business leader must look beyond the initial price tag and consider the Total Cost of Ownership (TCO). When you do this, the economic case for isononanoic acid becomes overwhelmingly strong.
Let’s break down the TCO:
- Regulatory & Compliance Costs: The costs associated with handling a Reprotoxic Category 1B substance are not trivial. They include specialized training, enhanced PPE, complex product labeling, and the administrative burden of tracking and reporting. These are real costs that eat into your margin. INA has none of this baggage.
- Performance & Maintenance Costs: In lubricant applications, the superior thermal stability of INA leads to longer fluid life. This means fewer oil changes, less downtime for machinery, and reduced labor costs. The extended life of the equipment itself due to better protection is an even larger, though harder to quantify, economic benefit.
- Risk & Liability Costs: This is the biggest hidden cost of using 2-EHA. What is the cost of a product recall if regulations tighten further? What is the potential liability of a lawsuit related to reproductive health? What is the cost of having to emergency-reformulate your entire product line when a ban is enacted? Using INA is a form of insurance against these catastrophic business risks.
- Brand & Marketing Value: In a competitive market, being able to promote your product as “Safer,” “Greener,” or “2-EHA Free” is a powerful differentiator that can command a premium price and build customer loyalty.
As a company that manufactures both 2-Ethylhexanoic Acid and Isononanoic Acid, we have a unique perspective 2. We see the market clearly shifting as formulators recognize that the small upfront premium for INA pays for itself many times over in safety, performance, and peace of mind.
Conclusion
The choice is clear. While 2-EHA was once a staple, its significant regulatory and safety issues, combined with its inferior thermal stability, make it a risky choice for new formulations. Isononanoic acid offers a safer, higher-performing, and future-proof alternative for lubricants, coatings, and beyond.
References
-
- 2-EHA Reclassification: exxonmobilchemical.com
-
- Carboxylic Acid Manufacturing: jytchem.com
-
- 2-Ethylhexanoic Acid: Wikipedia, The Free Encyclopedia.
-
- ECHA Substance Information on 2-EHA: European Chemicals Agency.